When intermolecular bonding of a liquid itself is substantially inferior to a substances surface it is interacting capillarity occurs. The volume is definite if.

Exposing a liquid to high temperatures would make it evaporate.
What are the characteristics of liquid. Characteristics Of Liquids 1. Liquids lack a definite shape so they acquire the one they print their container. A glass of water will have.
The fluidity is one of the main characteristics of liquids and gases which determines its ability to leave. Liquids consists of particles that are close together with no set arrangement can flow easily can take on the shape of any container it occupies and cannot be compressed easily. There are three main states of matter.
Solids liquids and gases. Liquids are considered the second state between solids and gases. Characteristics of Liquids They assume the shape of the container.
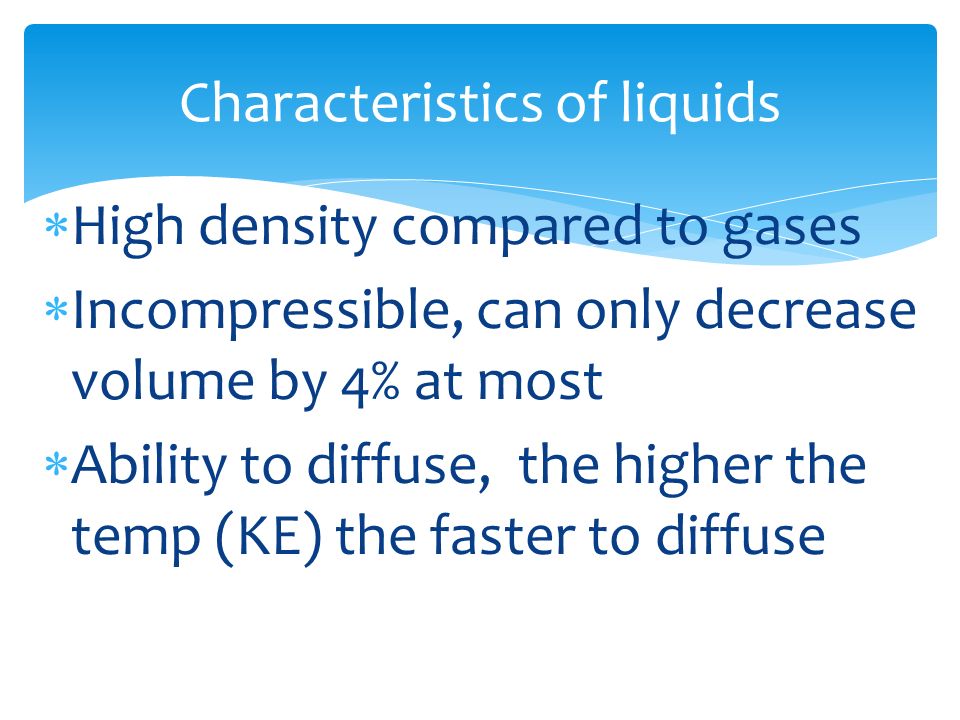
Their particles are loosely packed together hence they can be slightly compressed. Their particle possesses low energy for movement and rotational motion hence they can flow to an extent. A liquid is a nearly incompressible fluid that confirms to the shape of its container but retains a nearly constant volume independent of pressure.
The volume is definite if. The 3 characteristics of a liquid are as follows. A liquid has a definite volume.
A liquid does not have a definite shape. Some of the important properties of liquid are. A liquid has a definite volume.
A liquid has no definite shape and acquires the shape of the container. It can flow from a higher lever to a lower level. A liquid can diffuse into another liquid but this is much slower as compared to.
Mar 16 2014. All liquids show the following characteristics. Liquids are almost incompressible.
In liquids molecules are pretty close to each other. The molecules does not have lot of space between them. The molecules can not squeezed closer to one another.
When intermolecular bonding of a liquid itself is substantially inferior to a substances surface it is interacting capillarity occurs. Also the diameter of the container as well as the gravitational forces will determine amount of liquid raised. Cohesive and Adhesive Forces.
Liquidshave definite volume but indefinite shape 2. They are free to form droplets and puddles when they are not inside a container 3. Click here to get an answer to your question what are the characteristics of liquid.
What are the characteristics of liquid assets Liquid assets are equivalent assets or as good as cash. In other words they are assets that are quick and easy to convert into cash. The faster and easier the conversion the more liquid this asset is.
In accounting terms liquid assets are cash or cash equivalents that are easily convertible into. Liquid crystal phases are generally cloudy in appearance which means that they scatter light in much the same way as colloids such as milk. This light scattering is a consequence of fluctuating regions of non-uniformity as small groups of molecules form and disperse.
The anisotropy of liquid crystals causes them to exhibit birefringence. Main features of the liquid state 1- Compressibility. The limited space between its particles makes the liquid almost incompressible substance.
2- Changes of state. Exposing a liquid to high temperatures would make it evaporate. This critical point is called a.
It is the. What are the characteristics of liquid Four Arizona New Mexico Tennessee and Texas of the five study states integrate medical primary and acute community-based LTSS and institutional long-term care intermediate care and skilled nursing facilities. Three of the states Arizona Tennessee and Texas also include behavioral health services.
Three characteristics of metals are liquid solids and malleable. Which of the following is a list of assets from most liquid to least liquid. Houses are the most liquid assets.
High density as compared to same substance as a liquid or gas low rate of diffusion millions of times slower than in liquids may be crystalline or amorphous. LIQUID - read pages 315-317. Moderately strong intermolecular forces.
Fluid - particles slipslide past each other. The overall movement of suspended or dissolved particles resulting from the random movements of individual particles. Any material that flows and offers little resistance to change in shape when under pressure.
All liquids show the following characteristics. I Liquids are almost incompressible but less incompressible than solids.