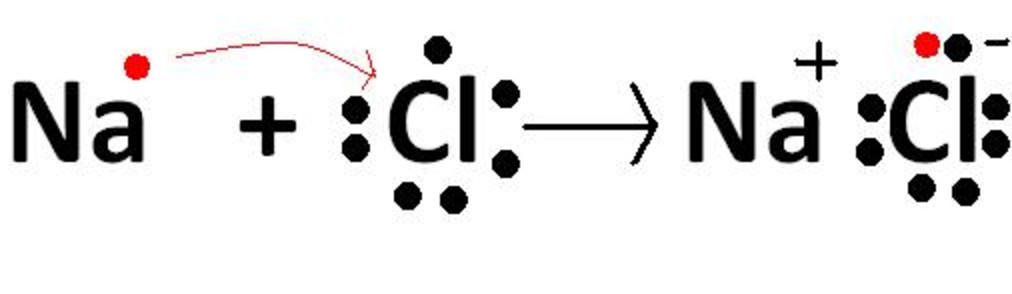
So sodium loses the single electron of the valence shell to form stable octet and chlorine gains one electron to form stable octet also. Due to opposite charges sodium and chloride ions are held together by the electrostatic force of attraction to form sodium chloride Na Cl or NaCl.
Thus both sodium and chlorine has 8 electrons in the valence shell.
How sodium chloride is formed. Due to opposite charges sodium and chloride ions are held together by the electrostatic force of attraction to form sodium chloride Na Cl or NaCl. Picture of formation of sodium chloride. The formation of sodium chloride can be shown more clearly with the help of a diagram as shown below.
Salt or sodium chloride is formed by neutralization of sodium hydroxide a base with hydrogen chloride an acid. Characteristics of a Sodium Chloride Sodium chloride is formed when sodium atoms interact with chlorine atoms. When this occurs sodium.
Its made when Na sodium and Cl chloride come together to form white crystalline cubes. Your body needs salt to function but too little or too much salt can be harmful to your health. How sodium chloride is formed.
Asked by Wiki User. Wiki User Answered 2014-12-30 190246. In laboratory sodium chloride is the product of a reaction between sodium.
Sodium chloride is obtained by mining the deposits and brine solution is obtained by passing water into the deposits. Hence the salts get dissolved then the solution is pumped out. Evaporation of the sea water is one of the major processes used to obtain salt and is most widely followed in countries like India.
Salt such as sodium chloride is formed when an acid and a base are neutralized in a chemical reaction. In nature sodium chloride commonly known as table salt is found when sea water evaporates. Additionally this salt can be mined from the Earth.
When sodium atom and chlorine atom come near each other sodium atom donates one electron to chlorine atom thereby forming singly charged positively sodium ion Na and singly negatively charged chloride ion Cl. Their electronic configuration resemble the electronic configurations of neon and argon respectively. Halite sodium chloride is found naturally in huge geologic deposits of salt minerals left over from the slow evaporation of ancient seawater.
Ever get a taste of seawater in your mouth at the beach These deposits are mined for various salts including enough sodium chloride to fill many many salt shakers. Sodium has a configuration of 281. Therefore chlorine has aconfiguration of 287.
So sodium loses the single electron of the valence shell to form stable octet and chlorine gains one electron to form stable octet also. Thus both sodium and chlorine has 8 electrons in the valence shell. Sodium chloride crystal is made up of sodium and chloride ions.
Sodium chloride crystal has a face-centered cubic close packed structureThe chloride ions o. Explain how sodium chloride is formed Ask for details. Follow Report by Ka4malho9tra 18102016 Log in to add a comment.
Due to opposite charges sodium ion and chloride ions are held together by the electrostatic force of attraction to form sodium chloride NaCl- or NaCl. Sodium Chloride is a metal halide composed of sodium and chloride with sodium and chloride replacement capabilities. When depleted in the body sodium must be replaced in order to maintain intracellular osmolarity nerve conduction muscle contraction and normal renal function.
Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds sodium hydroxide is used in soap manufacture and sodium chloride edible salt is a de-icing agent and a nutrient for animals including humans. Hydrochloric acid has the chemical formula HCl and you most definitely cannot drink it at concentrations above about 1M without receiving acid burns all over your alimentary canal.
Sodium chloride is formed when you react sodium metal or oxide with hydrochloric acid.