EOX for the treatment of Stomach Cancer. You have EOX chemotherapy as cycles of treatment.
Suitable neoadjuvant chemotherapy is important to treat patients with advanced gastric cancer.
Eox chemotherapy gastric cancer. EOX is used to treat stomach and oesophageal cancer. It is best to read this information with our general information about chemotherapy and the type of cancer you have. Your doctor will talk to you about this treatment and its possible side effects before you agree consent to have treatment.
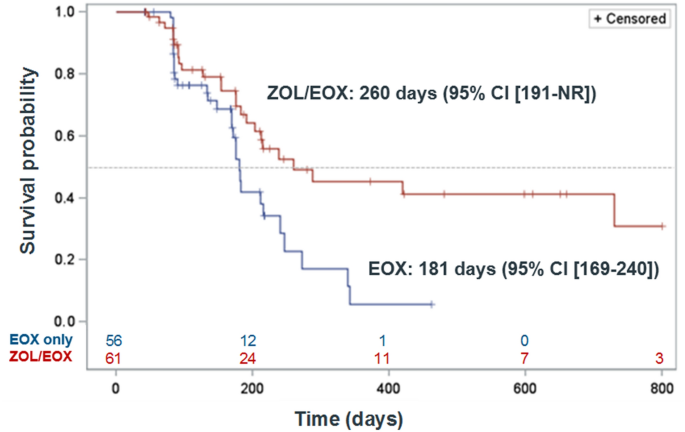
Patients pts with advanced HER2-negative HER GGEJ cancer with CLDN182 expression of 2 staining intensity with the anti-CLDN18 43-14A mAb in 40 tumor cells were eligible NCT01630083. Patients were randomized 11 to receive first-line EOX zolbetuximab loading dose 800 mgm 2 then 600 mgm 2 Q3W. Perioperative chemotherapy improves survival outcomes in locally advanced LA gastric cancer.
We retrospectively analyzed patients with LA gastric cancer who were offered perioperative chemotherapy consisting of epirubicin oxaliplatin and capecitabine EOX from May 2013 to December 2015 at Tata Memorial Hospital in Mumbai. Chemotherapy for Chinese patients with advanced gastric cancer. The EOX regimen may be suitable for younger patients subjected to individual neoadjuvant chemotherapy.
Introduction Gastric cancer is considered the third and fifth most common cause of cancer-related mortalities in males and females worldwide respectively. In addition gastric cancer ranks. Suitable neoadjuvant chemotherapy is important to treat patients with advanced gastric cancer.
FOLFOX and ECF including ECF-modified EOX are considered the main regimens in neoadjuvant chemotherapy. In China FOLFOX regimen is commonly used due to its effectiveness and high compliance 6 7. Patients with unresectable locally advanced gastric cancer were performed EOX regimen or XELOX regimen at the discretion of the investigators.
They were assessed for response every 2 cycles by CT computed tomography scan. A multidisciplinary team reassessed resectability after 4 cycles. The primary endpoint was the response rate.
Secondary end points included the R0 resection. My husband was diagnosed with metastatic gastric cancer just before christmas and was prescribed 6 cycles of EOX chemotherapy. After 3 cycles he had a CT scan which showed significant shrinkage of the lymph nodes the tumour in his stomach cannot be seen on a CT it was found doing a gastroscopy.
His tumour marker had also reduced a lot. The problem started on leaving the chemo. Paclitaxel capecitabine Category 2B 11.
Paclitaxel 4550mgm 2 IV. Capecitabine 625-825mgm 2 orally twice daily. Repeat cycle weekly for 5 weeks.
Epub 2012 Jun 30. Phase I study of 3-weekly combination chemotherapy using epirubicin oxaliplatin and S-1 EOS in patients with previously untreated advanced gastric cancer. Moehler M Shitara K Garrido M Nivolumab nivo plus chemotherapy chemo versus chemo as first-line 1L treatment for advanced gastric cancergastroesophageal junction cancer GCGEJCesophageal adenocarcino-ma EAC.
First results of the CheckMate 649 study Annals of Oncology 2020 31 suppl_4. When you have EOX. You have EOX chemotherapy as cycles of treatment.
A cycle of treatment means that you have a combination of drugs and then have a rest to allow your body to recover. Each cycle lasts 3 weeks 21 days. Depending on your cancer type you have between 6 and 8 cycles.
You have each cycle of treatment in the following way. EOX is most often associated with treatment of Esophageal Cancer and Gastric Cancer. Gastric cancer is the second most frequent cancer in the world.
Approximately 84 of patients with gastric cancer will have advanced disease and median survival of these patients without chemotherapy is only 3-4 months. Classical chemotherapy regimens mainly CF cisplatin plus infusional 5FU and ECF cisplatin plus infusional 5FU plus Epirubicin obtain responses in 20-40 of. New combination regimens such as docetaxel-cisplatin-fluorouracil DCF epirubicin-oxaliplatin-capecitabine EOX fluorouracil-leucovorine-oxaliplatin FLO irinotecan leucovorin and 5-FU ILF cispaltin plus xeloda S1 plus cisplatin are considered as new options for the first-line chemotherapy of advanced gastric cancer.
Patients with unresectable locally advanced gastric cancer were performed EOX regimen or XELOX regimen at the discretion of the investigators. They were assessed for response every 2 cycles by CT computed tomography scan. A multidisciplinary team reassessed resectability after 4 cycles.
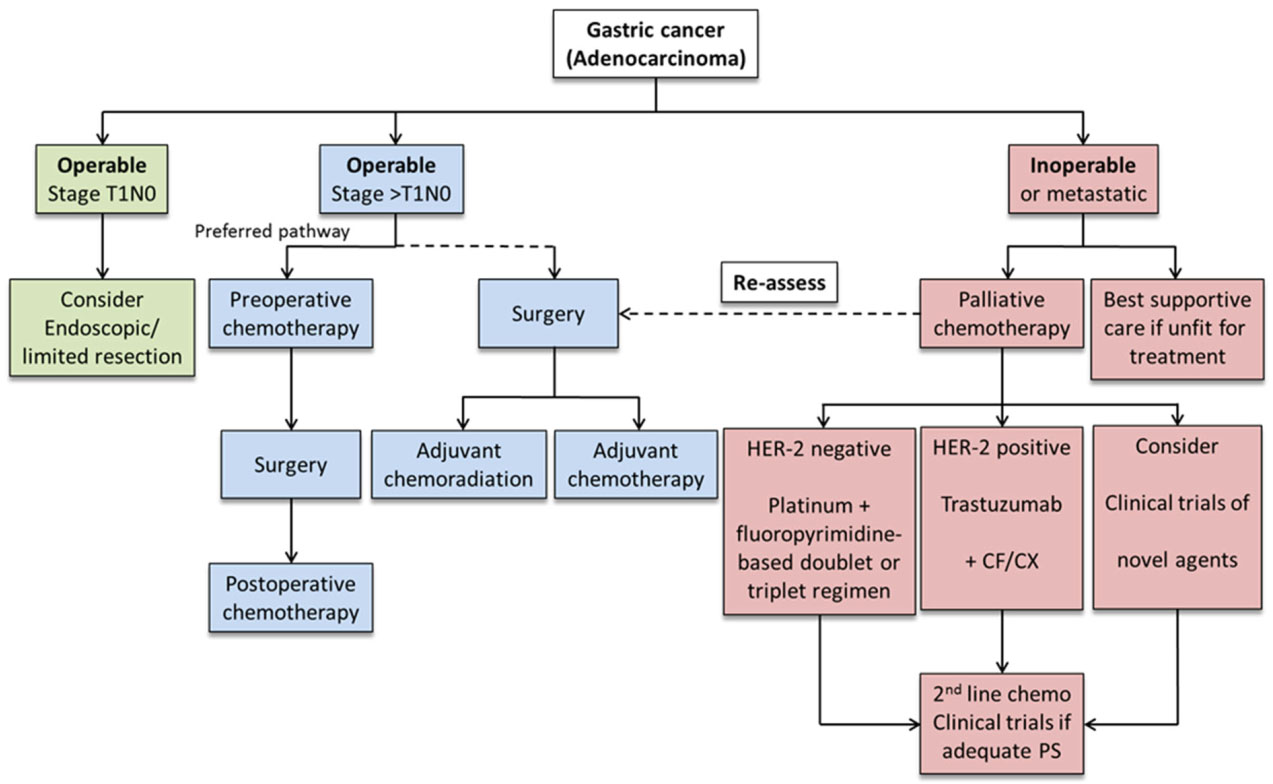
The primary endpoint was the response rate. In a review of the literature preoperative chemotherapy has been recommended as the preferred treatment pathway for advanced gastric cancer because of the advantages of tumor downstaging increased tumor resection rate and improved survival rate 18 20. How EOX chemotherapy is given and possible side effects.
EOX for the treatment of Stomach Cancer. EOX is the acronym for a chemotherapy regimen used in the treatment of stomach cancer E Epirubicin O Oxaliplatin X Xeloda capcitabine MOST COMMON SIDE EFFECTS OF EOX. Nausea Vomiting Risk of Infection Diarrhea Hand Foot Syndrome For more information see the Expert.
The value of postoperative three-cycle EOX regimen will be tested in patients with locoregionally advanced gastric cancer with positive pathological response to preoperative three-cycle EOX chemotherapy regimen. The patients will be randomized to the postoperative chemotherapy or to the follow-up arm. Participants will receive up to 8 cycles of epirubicin oxaliplatin and capecitabine EOX chemotherapy treatment alone 50 mgm2 epirubicin intravenously on day 1 of each cycle 130 mgm2 oxaliplatin intravenously on day 1 of each cycle 625 mgm2 capecitabine orally twice daily on days 1 to 21 of each cycle.
The first dose of capecitabine to be taken in the evening of day 1. Medical research Council adjuvant Gastric infusional Chemotherapy mit rund 500 Patienten wurde der Vor- teil einer perioperativen Chemotherapie mit einem Fluo- ropyrimidin einem Platinderivat und einem anthrazyklin beobachtet Cunningham d et al 2006 N Engl J Med 355. Allerdings hatte aufgrund von toxizitäten weniger als die Hälfte der Patienten die postoperative.