What is the effectiveness and safety of direct oral anticoagulants DOACs compared with warfarin in patients with valvular atrial fibrillation AF. The distinction between valvular and non-valvular AF remains a matter of debate.

35 The dabigatran dose was blinded whereas warfarin.
Dabigatran in valvular atrial fibrillation. Dabigatran the oral anticoagulant of choice at discharge in patients with non-valvular atrial fibrillation and COVID-19 infection. The ANIBAL protocol Drugs Context. Idarucizumab has recently been approved by the US Food and Drug Administration for the reversal of dabigatran.
6 Idarucizumab is a humanized monoclonal antibody fragment indicated in dabigatrantreated patients when reversal of the anticoagulant effects of dabigatran is needed for emergency surgery or urgent procedures and in lifethreatening or uncontrolled bleeding. 6 Idarucizumab received accelerated approval based on a reduction in unbound dabigatran. Direct oral anticoagulants DOACs are indicated by the European Medicines Agency and US Food and Drug Administration for stroke prevention in patients with nonvalvular atrial fibrillation AF.
The role of DOACs in patients with AF and concomitant valvular heart disease VHD is. In 2010 dabigatran etexilate a direct thrombin inhibitor was the first new oral anticoagulant to be approved for thromboembolic prophylaxis in atrial fibrillation in over 50 years. Dabigatran unlike warfarin has a short half-life with a rapid onset of anticoagulant effect does not require dose adjustment or monitoring and does not interact with food.
Dabigatran dose 2 hours prior to a dose of verapamil or quinidine. No dosage adjustment to dabigatran is recommended for patients concurrently taking these interacting medications for atrial fibrillation or VTE treatment but dose reduction to 150 mg once daily is recommended by the manufacturer for thromboprophylaxis in hip or knee replacement. We enrolled 121 consecutive non-valvular atrial fibrillation patients aged 60 years on dabigatran therapy mean age 796 74 years.
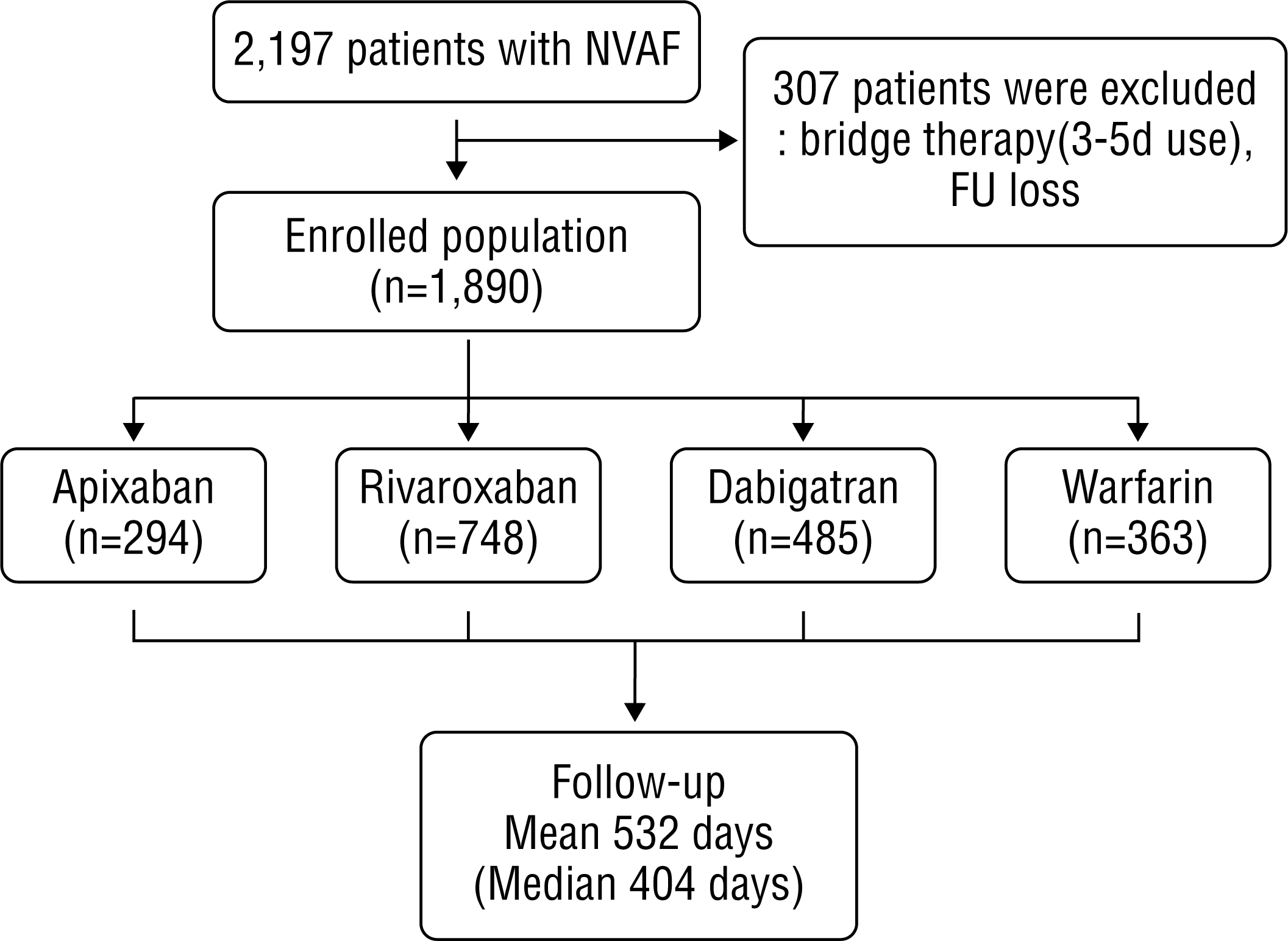
They were administered conventional low-dose dabigatran 110 mg twice daily or extended-interval dosing. Atrial fibrillation AF confers a substantial risk of stroke. Recent trials comparing vitamin K antagonists VKAs with non-vitamin K antagonist oral anticoagulants NOACs in AF were performed among patients with so-called non-valvular AF.
The distinction between valvular and non-valvular AF remains a matter of debate. Direct oral anticoagulants DOACs have been proven to be effective and safe for prevention of ischemic stroke and systemic embolism in patients with non-valvular atrial fibrillation NVAF. However suboptimal adherence variable dosing and use in patient populations that otherwise would have been excluded from clinical trials may impact the.
What is the effectiveness and safety of direct oral anticoagulants DOACs compared with warfarin in patients with valvular atrial fibrillation AF. The investigators conducted a new-user retrospective propensity scorematched cohort study using a US-based commercial health care database from January 1 2010June 30 2019. Reduction of risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation.
Treatment of deep venous thrombosis and pulmonary embolism in patients who have been treated with a parenteral anticoagulant for 5 to 10 days. Reduction in the risk of recurrence of deep venous thrombosis and pulmonary embolism in patients. Dabigatran is an oral drug used to treat and prevent blood clots an anticoagulant or blood thinner in the hearts of patients with atrial fibrillation.
These clots are likely to break into pieces and travel to the brain to cause strokes. Dabigatran has been compared with warfarin in patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke. 5 The RE-LY study randomised more than 18000 patients at risk of stroke mean CHADS 2 score 21 to dabigatran 150 mg twice daily dabigatran 110 mg twice daily or dose-adjusted warfarin.
35 The dabigatran dose was blinded whereas warfarin. Dabigatran rivaroxaban and apixaban received Norwegian marketing authorization for non-valvular atrial fibrillation in August 2011 December 2011 and November 2012 respectively. 15 July 2013 was chosen as start date of this study because this was when apixaban as the last study drug received general reimbursement for treatment of atrial fibrillation and reimbursement codes were.
Importance Dabigatran and rivaroxaban are nonvitamin K oral anticoagulants approved for stroke prevention in patients with nonvalvular atrial fibrillation AF. There are no randomized head-to-head comparisons of these drugs for stroke bleeding or mortality outcomes.